Glossary
Proton Emission
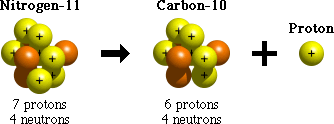
Proton emission is one process that unstable atoms can use to become more stable. During proton emission, a proton is ejected from an atom's nucleus.
Since an atom loses a proton during proton emission, it changes from one element to another. For example, after undergoing proton emission, an atom of nitrogen (with 7 protons) becomes an atom of carbon (with 6 protons).
Citation and linking information
For questions about this page, please contact Steve Gagnon.
