Glossary
Alpha Decay
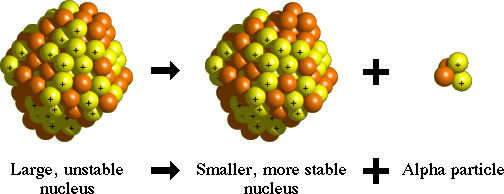
Alpha decay is one process that unstable atoms can use to become more stable. During alpha decay, an atom's nucleus sheds two protons and two neutrons in a packet that scientists call an alpha particle.
Since an atom loses two protons during alpha decay, it changes from one element to another. For example, after undergoing alpha decay, an atom of uranium (with 92 protons) becomes an atom of thorium (with 90 protons).
Citation and linking information
For questions about this page, please contact Education Web Administrator.
