Glossary
Electron Capture
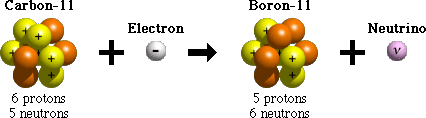
Electron capture is one process that unstable atoms can use to become more stable. During electron capture, an electron in an atom's inner shell is drawn into the nucleus where it combines with a proton, forming a neutron and a neutrino. The neutrino is ejected from the atom's nucleus.
Since an atom loses a proton during electron capture, it changes from one element to another. For example, after undergoing electron capture, an atom of carbon (with 6 protons) becomes an atom of boron (with 5 protons).
Although the numbers of protons and neutrons in an atom's nucleus change during electron capture, the total number of particles (protons + neutrons) remains the same.
Electron capture is also called K-capture since the captured electron usually comes from the atom's K-shell.
Citation and linking information
For questions about this page, please contact Education Web Administrator.
