Glossary
Half-life
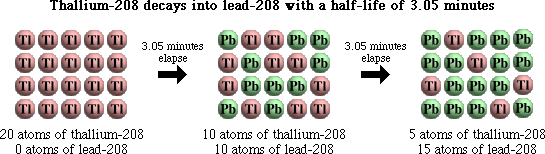
The half-life describes the amount of time needed for half of a sample of unstable atoms or particles to undergo decay. Thallium-208, for example, decays into lead-208 with a half-life of 3.05 minutes. This means that half of a sample of thallium-208 will decay into lead-208 over the course of 3.05 minutes.
Scientists cannot predict when a particular atom or particle will decay. They only know that, on average, half of a sample will decay during the span of one half-life.
Citation and linking information
For questions about this page, please contact Education Web Administrator.
