Questions and Answers
How do I read an electron configuration table?
Are you making a model of an atom and need to know how to place the electrons around the nucleus? If so, you will need to know how to read an element's electron configuration table. Follow these easy directions to learn how!
What is an electron configuration table?
An electron configuration table is a type of code that describes how many electrons are in each energy level of an atom and how the electrons are arranged within each energy level. It packs a lot of information into a little space and it takes a little practice to read. For example, this is the electron configuration table for gold:
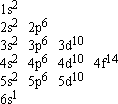
What do all those numbers and letters mean?
Each row of an electron configuration table is sort of like a sentence. Each 'sentence' is made up of smaller 'words'. Each 'word' follows this format:
![]()
The first number is the energy level. We can tell right away that an atom of gold contains 6 energy levels.
The lowercase letter is the sub-shell. The sub-shells are named s, p, d and f. The number of available sub-shells increases as the energy level increases. For example, the first energy level only contains an s sub-shell while the second energy level contains both an s sub-shell and a p sub-shell.
The number in superscript is the number of electrons in a sub-shell. Each sub-shell can hold only a certain number of electrons. The s sub-shell can hold no more than 2 electrons, the p sub-shell can hold 6, the d sub-shell can hold 10 and the f sub-shell can hold as many as 14.
How can I use the electron configuration table to tell me...
How many energy levels does an atom have?
Since the electron configuration table lists each energy level by row, you can tell how many energy levels there are by seeing how many rows there are. As was mentioned earlier, an atom of gold contains six energy levels, as shown below:
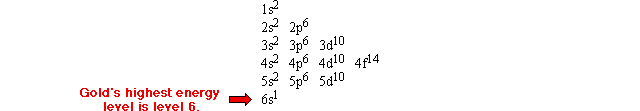
How many electrons are in each energy level?
The total number of electrons in an energy level is the sum of the electrons in each sub-shell of that energy level. Just add the numbers in superscript together to find the number of electrons in an energy level. The number of electrons in each energy level in an atom of gold is shown below:
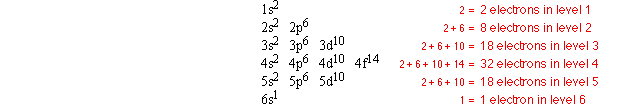
How many electrons are in an atom's outer energy level?
This is just a combination of the previous two examples. Use the electron configuration to find that atom's highest energy level and then add up the numbers in superscript to find the number of electrons that are in it. There is one electron in the outer energy level of an atom of gold, as shown below:
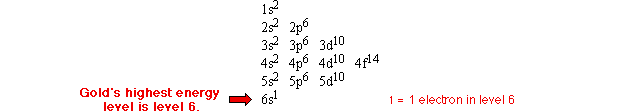
Citation and linking information
For questions about this page, please contact Carol McKisson.
