Questions and Answers
How much of an atom is empty space?
Very nearly all of it. Let's take a look at an atom of hydrogen to see how empty it really is. Of course, this diagram isn't drawn to scale...
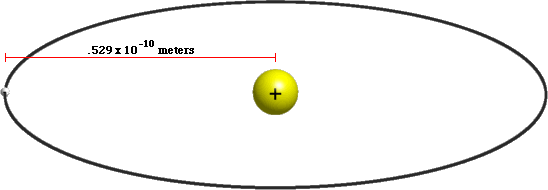
A hydrogen atom is made from a single proton that's circled by a single electron. How big is a hydrogen atom? The radius of a hydrogen atom is known as the Bohr Radius, which is equal to .529 × 10-10 meters. That means that a hydrogen atom has a volume of about 6.2 × 10-31 cubic meters.
How big is the proton at the center of a hydrogen atom? Recent studies indicate that protons have a radius of about .84 × 10-15 meters, giving them a volume of about 2.5 × 10-45 cubic meters.
We need to do a little more math to find out how much of a hydrogen atom is empty space:
Percent Full = 100 × (Volume Filled / Total Volume)
Percent Full = 100 × (2.5 × 10-45 m3 / 6.2 × 10-31 m3)
Percent Full = 100 × (4 × 10-15)
Percent Full = 4 × 10-13 %
Percent Full = 0.0000000000004%
If 0.0000000000004% of a hydrogen atom is full, then the rest of it must be empty:
Percent Empty = 100% - Percent Full
Percent Empty = 100% - 0.0000000000004%
Percent Empty = 99.9999999999996%
A hydrogen atom is about 99.9999999999996% empty space. Put another way, if a hydrogen atom were the size of the earth, the proton at its center would be about 200 meters (600 feet) across. While I wouldn't want something that big landing on my head, it's tiny compared to the size of the earth.
Citation and linking information
For questions about this page, please contact Education Web Administrator.
