Glossary
Neutron Emission
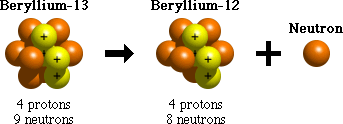
Neutron emission is one process that unstable atoms can use to become more stable. During neutron emission, a neutron is ejected from an atom's nucleus.
Since the number of protons within an atom doesn't change during neutron emission, it doesn't change from one element to another. It does, however, become a different isotope of that element. For example, after undergoing neutron emission, an atom of beryllium-13 (with 9 neutrons) becomes an atom of beryllium-12 (with 8 neutrons).
Citation and linking information
For questions about this page, please contact Carol McKisson.
